Perforin-2 and Innate Immunity
Perforin-2, the product of the Mpeg1 gene, is a major focus of the lab. Beginning with the foundational work of Dr. Eckhard Podack, our former Chair and colleague, studies at the University of Miami have shown that bacterial pathogens are able to survive and even proliferate within macrophages that lack Perforin-2.
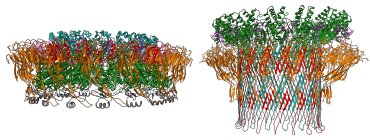
Perforin-2 is an effector of the innate immune system that limits the spread and proliferation of bacterial pathogens. Its amino-terminus contains a Membrane Attack Complex Perforin (MACPF) domain which is also present in several complement proteins and Perforin. Gram-negative bacteria are killed when complement proteins C6-C9 form pores in the bacterial envelope. Cytotoxic T lymphocytes (CTL) and natural killer cells (NK) use Perforin to form lytic pores in the membranes of tumor and virally infected mammalian cells. In a 2020 publication our collaborator Prof. Robert Gilbert demonstrated that Perforin-2 also forms pores by solving the structure of the Perforin-2 pre-pore and pore complexes. In a separate study we showed that Perforin-2 is required to breach the envelope of phagocytosed bacteria. In contrast to complement C6-C9 whose activity is restricted to gram-negative bacteria, the bactericidal activity of Perforin-2 is broad spectrum; efficacious against gram-negative, gram-positive, and acid-fast bacteria. Moreover, most mammalian cells have the capacity to express Perforin-2 and this ability endows both immune and non-immune, tissue forming cells with a frontline defense against pathogenic bacteria. Not surprisingly, Perforin-2 deficient mice are severely immunocompromised and rapidly succumb to infectious diseases. We are also working with clinicians who have discovered patients with deleterious Mpeg1 mutations that correlate with increased susceptibility to infectious diseases. To better understand the pivotal role of Perforin-2 within the innate immune system we are using multidisciplinary approaches to elucidate its activation, intracellular trafficking, mechanisms of pore-formation and killing, and crosstalk with other parts of the immune system.
Anti-Perforin-2 Bacterial Effectors
Further illustrating Perforin-2’s ancient yet pivotal role in host defense, we have discovered that evolution has endowed microbial pathogens with effectors that block Perforin-2’s bactericidal activity. For example, we have shown that enteropathognic E. coli (EPEC) and Yersinia pseudotuberculosis inject an enzyme, Cif, into the cytosol of host cells to block the trafficking of Perforin-2. Thus, Perforin-2 is unable to reach the phagosome. We believe that relief of these roadblocks would restore Perforin-2 activity and allow a patient’s own cells to successfully clear many types of bacterial infections. Ultimately this may provide a solution to the scourge of antibiotic resistance. To this end we are using multidisciplinary approaches to discover and characterize bacterial effectors that subvert or inhibit the bactericidal activity of Perforin-2 with a particular emphasis on enteric and multidrug resistant pathogens.
Diarrheagenic E. coli and other enteric pathogens
Approximately 1.7 million people, primarily infants and children, perish from diarrheal diseases every year. For citizens of low–income countries diarrheal diseases are among the ten leading causes of death. Although many viral and bacterial pathogens cause diarrhea, enterotoxigenic E. coli (ETEC) is prevalent in low–income nations where it is estimated to kill between 300,000 to 700,000 children and infants each year. Another 280 million people are sickened by ETEC annually including travelers from high income countries. Despite decades of research there are no FDA approved vaccines to prevent ETEC infections. Although some vaccines are currently under development and evaluation, at least one initially promising vaccine has failed in a large scale clinical trial. Genome plasticity and strain heterogeneity is a further obstacle to vaccine development. Thus, the prospects for ETEC vaccines are uncertain.