Perforin-2 (MPEG1) plays a pivotal role in host defense
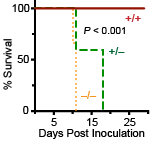
The phagocytosis and destruction of microorganisms is an essential immunological function of macrophages and other phagocytes that protects us from invading pathogens. Our research at the University of Miami and with collaborators at other institutions has shown that Perforin-2 (MPEG1) is essential for the destruction of phagocytosed microbes. Although macrophages were first described by Elie Metchnikoff in the 19th century, Perforin-2’s pivotal role within macrophages was not discovered until the 21st century. Beginning with the foundational work of our former Chair and colleague Dr. Eckhard Podack studies at the University of Miami have shown that bacterial pathogens are able to survive and even proliferate within macrophages that lack Perforin-2. As expected from cell based studies, Perforin-2 knockout mice succumb to a variety of bacterial pathogens at doses that the majority of their wild-type littermates survive. This is accompanied by replication and dissemination of bacteria to deeper tissues. Further underscoring the importance of Perforin-2 in host defense is the fact that some pathogens deploy effectors to block the delivery of Perforin-2 to phagosomes.
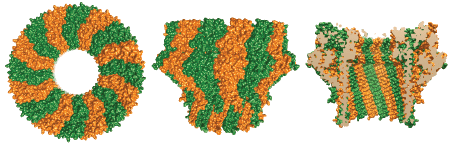
Structures of Perforin-2 polymers
Like C9 and Perforin -two extensively characterized pore forming proteins of our immune system- Perforin-2 has a membrane attack complex perforin domain; commonly abbreviated as “MACPF.” With the assistance of other complement proteins circulating in our serum, C9 perforates the envelopes of gram-negative bacteria. Perforin is deployed when cytotoxic T lymphocytes or natural killer cells degranulate to destroy virally infected or cancerous cells. C9 and Perforin polymerize into rings with each MACPF domain deploying four amphipathic beta-strands. Each set of four strands aligns with those of neighboring subunits within the ring. Thus the deployed beta-strands, which were alpha-helical prior to deployment, form the barrel of the pore through target lipid bilayers.
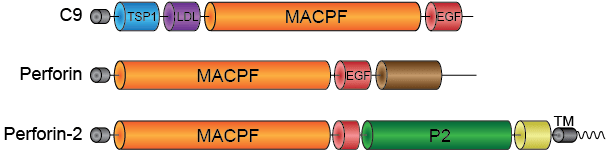
The presence of a MACPF domain within Perforin-2 gave rise to the hypothesis that it too was a pore-forming protein. Recent high resolution structures of Perforin-2 by our collaborator Prof. Robert Gilbert at the University of Oxford have shown that this is indeed the case. Perforin-2 polymerizes into rings of 16 subunits. MACPFs form an inner ring while the enigmatic P2-domains line the outer ring. In the pre-pore the residues that will be deployed to form the barrel of the pore are retracted in their helical conformation. Our collaborators also discovered that the transition from pre-pore to pore is driven by low pH. The discovery that acid is the trigger for pore formation fits well with the role of Perforin-2 within phagosomes because the lumen of phagosomes is acidic. Remarkably the pre-pore to pore transition also involves a 180° rotation of the MACPF relative to the P2 domain.
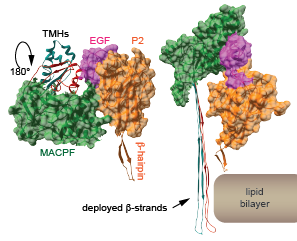
Structural and functional studies have determined that the P2 domain contains an extended beta-hairpin that is involved in the initial interactions with target membranes.
Unexpectedly, high resolution structures have also revealed that the pre-pore to pore transition involves a 180° reorientation of the MACPF relative to the P2 domain and is driven by low pH. In situ this transition likely occurs during phagosome acidification. The transmembrane hairpins (TMHs) within the MACPF are primarily alpha-helical in the pre-pore but transition to extended beta-strands to form the pore. The rendered subunits were extracted from the pre-pore and pore oligomers in PDB files 6SB3 and 6SB5.
Selected Publications
- Podack ER, Munson GP. Killing of Microbes and Cancer by the Immune System with Three Mammalian Pore-Forming Killer Proteins. Frontiers in Immunology. 2016;7:464. Review. PMID: 27857713, PMCID: PMC5093134.
- McCormack RM, Lyapichev K, Olsson ML, Podack ER, Munson GP. Enteric pathogens deploy cell cycle inhibiting factors to block the bactericidal activity of Perforin-2. Elife. 2015 Sep 29;4. PMID: 26418746, PMCID: PMC4626573.
- McCormack RM, de Armas LR, Shiratsuchi M, Fiorentino DG, Olsson ML, Lichtenheld MG, Morales A, Lyapichev K, Gonzalez LE, Strbo N, Sukumar N, Stojadinovic O, Plano GV, Munson GP, Tomic-Canic M, Kirsner RS, Russell DG, Podack ER. Perforin-2 is essential for intracellular defense of parenchymal cells and phagocytes against pathogenic bacteria. Elife. 2015 Sep 24;4. PMID: 26402460, PMCID: PMC4626811.
- Bai F, McCormack RM, Hower S, Plano GV, Lichtenheld MG, Munson GP. Perforin-2 Breaches the Envelope of Phagocytosed Bacteria Allowing Antimicrobial Effectors Access to Intracellular Targets. The Journal of Immunology. 2018 Nov 1;201(9):2710-2720. PMID: 30249808, PMCID: PMC6200583.
- Ni T, Jiao F, Yu X, Aden S, Ginger L, Williams SI, Bai F, Pražák V, Karia D, Stansfeld P, Zhang P, Munson G, Anderluh G, Scheuring S, Gilbert RJC. Structure and mechanism of bactericidal mammalian perforin-2, an ancient agent of innate immunity. Science Advances. 2020 Jan;6(5):eaax8286. PMID: 32064340, PMCID: PMC6989145.