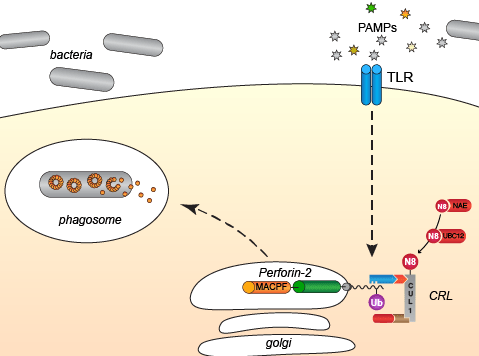
Although Perforin-2’s carboxy-terminal transmembrane domain and cytosolic tail are just a small fraction of the total protein, they both have essential functions. For example, deletion of its transmembrane domain results in the secretion of Perforin-2 to the extracellular milieu (Ni et al. 2020, Pang et al. 2019). Thus, its transmembrane domain is essential for the intracellular retention of Perforin-2 and its sequestration in non-acidic compartments until it is needed within phagosomes. The short 38-39 amino acid cytosolic tail of Perforin-2 is also key to maintenance of its inactive, latent state until it is needed to kill phagocytosed bacteria. PAMPs, such as LPS or flagellin of invading bacteria, trigger monoubiquitylation of one to four conserved lysine residues in its cytosolic tail by a cullin-RING E3 ubiquitin ligase (CRL). As with other proteins, monoubiquitylation –or poly monoubiquitylation– of PRF2 is an intracellular sorting signal that directs Perforin-2 from one subcellular compartment to another. In Perforin-2’s case ubiquitylation of its cytosolic tail results in the rapid redistribution of Perforin-2 from diffuse perinuclear regions to distinct endo-phagosomes.
Cullins such as CUL1 form the CRL scaffold and cullin neddylation drives ubiquitylation of the CRL substrate; in this case the cytosolic tail of Perforin-2. We have shown that siRNA knockdown of CUL1 or the NEDD8 conjugating enzyme UBC12 abolishes ubiquitylation of Perforin-2 and Perforin-2-dependent killing of phagocytosed bacteria (McCormack et al. 2015). Likewise, mutagenesis of the conserved lysine residues in its cytosolic tail abolish ubiquitylation, trafficking, and Perforin-2 dependent killing (McCormack et al. 2015).
Based on these and other observations our current working hypothesis is that a protein or series of proteins recognize that Perforin-2 has been ubiquitylated and subsequently deliver Perforin-2 to endo-phagosomes. Subsequently, a protease releases Perforin-2 from its transmembrane domain. Once freed from its membrane tether, which may also serve to inhibit polymerization and pore formation, Perforin-2 deposits and polymerizes on the envelope of the phagocytosed bacterium. Acidification of the phagosome triggers the transition from pre-pore to pore thus facilitating delivery of proteases and other antimicrobials to the interior of the bacterium.
Current Questions:
- Do PAMPs drive phosphorylation of Perforin-2? If so, what is the kinase and how is it activated?
- How does the CRL recognize Perforin-2 at the appropriate time? Is phosphorylation required for ubiquitylation?
- What are the proteins, or series of proteins, that recognize ubiquitylated Perforin-2 and deliver it to endo-phagosomes?
- When, where, and how is Perforin-2 cleaved from its transmembrane domain? Is a specific protease involved?