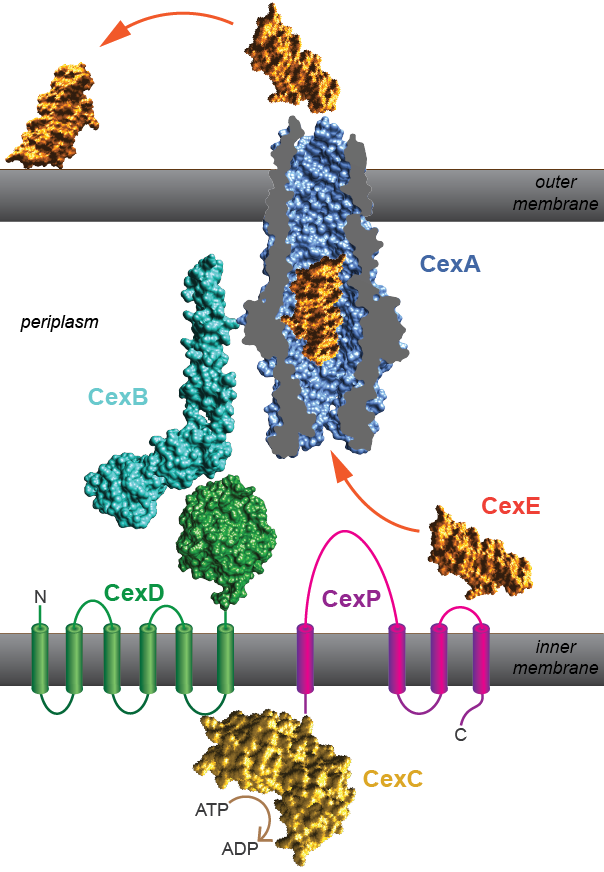
Canonical Type 1 Secretion Systems (T1SS) of gram-negative bacteria accept client proteins from the cytoplasm and transport them across the inner membrane, through the periplasm, and finally across the outer membrane in a single translocation step. The prototypical T1SS is the hemolysin system of E. coli that exports hemolytic toxin (Thomas, Holland and Schmitt 2014). All five Cex proteins, CexPABCD, are required for the secretion of CexE to the outer membrane and some are homologous to components of T1SS. Most notable is CexA which is a member of the TolC family. As has been shown for TolC, CexA likely forms a trimeric beta-barrel through the outer membrane. T1SS also have ATP binding cassettes (ABCs) and hydrolyze ATP to drive protein secretion. In the Cex system the ABC resides within CexC and it is the putative ATPase of the secretion system.
However the Cex secretion system is a noncanonical T1SS because it accepts its client protein -CexE- from the periplasm, not the cytoplasm. We have shown that CexE has an amino-terminal signal peptide (Pilonieta, Bodero and Munson, 2007) and is transported across the inner membrane by the SecYEG translocase. As it enters the periplasm its signal peptide is removed by a signal peptidase. What happens next to CexE is not yet clear. The protein may undergo further maturation; in particular the formation of an internal disulfide bond and additional post-translational modifications. Eventually the Cex secretion accepts periplasmic CexE for final transport across the outer membrane. We have chosen to designate this system as a Type1p Secretion System (T1pSS) because the Cex secretion system is noncanonical. Here “p” denotes that this system accepts a periplasmic client.
Current Questions:
- How does the Cex secretion system recognize its client?
- Do the periplasmic domains of CexD or CexP bind CexE or other secretion components?
- Is post-translational modification of CexE required for secretion?
- How is the hydrolysis of ATP by cytoplasmic CexC coupled to the secretion of CexE?
- Is secretion regulated by specific external or internal stimuli?
- What is the stoichiometry of the assembled secretion system?
- Does the secretion system assemble in the absence of CexE?
- Does CexE change the stoichiometry of the secretion system or its intermolecular associations?