All antibodies contain both heavy and light chains.
As students of immunology most of us were –and many still are– taught that immunoglobulins are composed of heavy and light chains. But in the late 1980s a group of biology students at the University of Brussels made a remarkable discovery that smashed this dogma. While isolating and analyzing antibodies from camel serum the students did indeed observe the expected repertoire of IgG antibodies with both heavy and light chains. Unexpectedly they also observed a subset that did not correlate with any known class of antibodies. Subsequent investigations by Hamers-Casterman et al. (Nature 1993) revealed that this new class of antibodies were devoid of light chains and are present in all camelids; including llamas and alpacas. This was an astonishing discovery because the paratope (antigen binding site) of “conventional” antibodies is formed by the synergistic participation of a heavy and light chain. To put it another way, an immunoglobulin without a light chain is not an antibody. Or at least that was the dogma until a group of university students made a serendipitous discovery in 1989. In this astonishing class of antibodies the paratope resides within the variable region of a single heavy chain polypeptide.
A subsequent study by researchers from our own Department of Microbiology and Immunology at the University of Miami School of Medicine found that sharks, and other cartilaginous fish, also produce heavy chain only antibodies. These antibodies, which evolved independently from those of camelids, are referred to as immunoglobulin new antigen receptor (IgNAR).
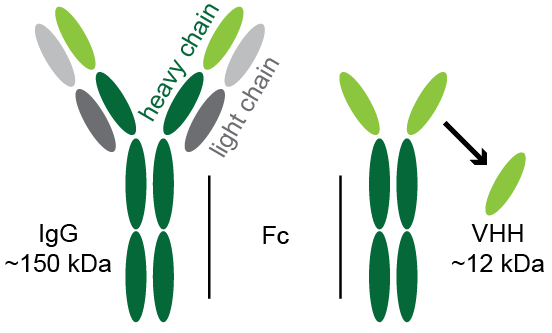
Today this new class of antibodies are referred to as VHHs, sdAbs, llama-bodies, or nanobodies. VHH -an acronym for Variable Heavy domain of Heavy chain- is the most technically precise term. sdAb is an acronym for Single Domain Antibodies. Llama-bodies is in reference to llamas as the preferred animal for raising VHHs against inoculated antigens. Nanobodies refers to the fact that the constant regions are dispensable since there is no need to pair a heavy chain with a light chain. Thus, the smallest functional unit of this new class of antibodies is ~12 kDa; less than one tenth the size of conventional IgGs. Moreover, once the sequence of a VHH is obtained the antibody can be conveniently mass produced in bacteria such as E. coli; eliminating the tedious effort and expense required for the production of mAbs by culture of mammalian hybridomas. The ability to produce VHHs in bacteria has most recently given rise to rationally designed, fully synthetic libraries of VHHs. Such synthetic libraries can be panned for VHHs with specificity to any given antigen; completely eliminating research animals (llamas) from the production protocol.
VHHs against Perforin-2
In collaboration with Hybribody and Prof. Gilbert the Munson lab is producing synthetic VHHs against Perforin-2. We are particularly interested in VHHs that can distinguish between three different Perforin-2 confimations:
- monomeric Perforin-2
- Perforin-2 pre-pores
- Perforin-2 pores
We are producing VHHs in E. coli and are in the process of validating them in various immunological assays; i.e., FLOW, IF, pull-downs, etc. In addition to our own research needs, our long term goal is to provide validated VHHs to the research community.